Weekend Update #255
Thank you for your continued support and engagement. Each week, we're sharing what companies we're researching and the what, the who and the how that we think makes the companies interesting and unique. This roundup is brought to you weekly by a group of interns, creative minds, artists and investors who believe that through best in class investing along with the democratization of financial education we can do great things together. Enjoy, Explore and Share.With the market past the majority of corporate earnings this week, investors shifted focus to the government shutdown, the Federal Reserve, and delayed economic data releases. Looking for directional signals, equity markets ended the week relatively flat with higher volatility. Investors began the week expecting an end to the government shutdown, and the House delivered a 222-209 vote to resolve the shutdown on Wednesday night. The bill includes funding for the Department of Agriculture, the Department of Veterans Affairs, the FDA, military construction projects, and Congress. The bill will fund the government through January 30th, with another vote to come mid-December to extend Affordable Care Act tax credits. Following the vote, the reality of up to a 2.0% drag on Q4 2025 GDP as a result of the 43-day shutdown, the low probability that economists will see key CPI and jobs data from October, and the risk of backlogged economic data trending in the wrong direction all contributed to a late-week selloff.
In economic data for the week, ADP’s weekly employment report showed the U.S. lost 11,250 jobs on average for the four weeks ended October 25th. This follows the October Challenger report that showed U.S. firms announced 153,074 job cuts in the month, the highest since 2003. NFIB’s Small Business Optimism report for October missed economists’ estimates, driven by lower profits in the month and deteriorating expectations for business conditions. Fitch Ratings also reported this week that 6.65% of subprime borrowers are 60 days or more past due on auto loans, the highest share since 1994 and revealing growing pressure on the riskiest borrowers.
Although there was a lack of economic data during the week, the BLS announced on Friday that it would provide the September jobs report on November 20th. As ADP data shows employment deteriorating into the end of October, the September report will be a welcome data point but could be viewed as outdated information. With the Federal Reserve operating under lower visibility given the delayed data, several FOMC members this week stated that inflation is of greater concern than employment, preferring to move more slowly on rate cuts. The more hawkish Fed speak has led to a lower probability of a December rate cut. The market is now implying roughly a 50/50 chance of a 25 basis point cut at the next meeting, down from 100% in mid-October.
Tech valuation concerns have come into play during the latest market volatility spike, so investors will look to Nvidia’s earnings call on Wednesday, November 19th, to gauge the balance between booming AI investment and its durability to inform valuation. In November, the ratio of the equal-weight S&P to the S&P 500 index fell to a low since 2003, showing investors still have confidence that the largest companies will dominate on AI-driven profits. Delayed releases of economic data and Nvidia’s commentary should serve as good indicators for these key market themes.
Friday’s Close (Weekly Performance)
S&P 500 6,734.11 (+0.08%)
Nasdaq 22,900.59 (-0.45%)
Dow Jones 47,147.48 (+0.34%)
Thank you Senior Analyst JARED FENLEY.
Precision BioSciences is an advanced gene editing company utilizing their novel proprietary ARCUS platform to develop in vivo gene editing therapies for sophisticated edits, including gene elimination, insertion and excision. ARCUS is the only gene editor derived purely from a protein, called a homing endonuclease, that evolved in nature to safely edit a genome and add function. ARCUS is particularly efficient at generating defined outcomes due to predominant repair using homology directed repair (HDR) as opposed to non-homologous endjoining (NHEJ).
Precision BioSciences held its initial public offering on March 27, 2019 at $16.00 per share. Adjusted for the 1:30 reverse split on February 16, 2024, shares have lost 99% of their value based on the $4.50 closing price on January 8, 2025. Currently, at $4.50 per share, the company has a market capitalization of $35 million, compared to the cash on the balance sheet of $109 million, or $14 per share.
In 2020, the Nobel Prize in Chemistry was awarded to Jennifer Doudna and Emmanuelle Charpentier for their groundbreaking work on CRISPR, discovering how to create genetic scissors which could unlock novel human therapeutics using CRISPR-Cas based gene editing technology. Today, there are three publicly traded companies using CRISPR to develop therapeutics, and they are worth billions of dollars in market capitalization.
In the near future, the scientific community, as well as the financial markets, may shine their light on Jeff Smith, Ph.D., Co-Founder and Chief Research Officer for Precision BioSciences, and the inventor of ARCUS, the company’s novel and proprietary gene editing technology. The key to ARCUS was found in the chloroplast genome of a single cell algae called Chlamydomonas reinhardtii, the perfect homing endonuclease, called I–CreI. Over billions of years, I-CreI has evolved to recognize a specific 22 nucleotide sequence, whose high specificity made it an attractive choice to build customizable gene targeting technology called ARCUS.
Daniel Levine: Joe, thanks for joining us.
Joe Truitt: Thanks, Danny. Thanks for the invite.
Daniel Levine: We’re going to talk about iECURE, its efforts to develop in vivo gene editing to treat and potentially cure liver diseases, and the opportunities for this approach. iECURE is built on a collaboration with James Wilson of the University of Pennsylvania Gene Therapy Program. What’s the relationship? Are you just commercializing technology developed there or is there ongoing interaction and collaboration with the GTP?
Joe Truitt: Thanks for that question, Danny. It’s fascinating how the company came to be. The organization of iECURE was really built around work that Jim Wilson and the Gene Therapy Program at Penn have been working on for multiple years. So as a new company, we actually have some pretty extensive data sets dating back five years when Jim had started this work. As this data matured, it became very apparent that this was going to be an attractive data set and something that should certainly be investigated for clinical development. So, the company was formed around the data and the way that the company is structured, interestingly, is that—typically in other pharmaceutical companies or biotech companies that I’ve worked in or ran we would have our own research engine where we had our own biologists and chemists and toxicologists, but in this case, the way we’ve structured, this agreement is the Gene Therapy Program and the Wilson lab will work on all the preclinical work, the toxicology, the early stage manufacturing, the early regulatory operations and submissions, and will take the programs all the way up to the IND phase where the baton will be handed off. And this is somewhat different than most academic collaborations where Jim’s team, with all of their experience and their hundreds of talented employees, are taking the programs a little bit further than academic institutions traditionally have. So it ends up being very beneficial for the company where I don’t have to go out and hire and build this whole infrastructure. So I can leverage the collaboration where Jim’s team will do all the work. They’ll hand the baton off at the IND phase, and then iECURE will be responsible for everything from the IND forward, which includes all the clinical development and commercialization.
Interview: Dr. Jeff Smith, Founder, Chief Research Officer, Precision BioSciences
Interview by Jonah Comstock, Pharmaphorum
J.P. Morgan Healthcare Conference
January 9—10, 2024
______________________________________________________________________________________________
Jonah Comstock
Hi. I am Jonah Comstock at Pharmaphorum Media Limited. I'm here on the sidelines of the J.P. Morgan 2024 Healthcare Conference at The Marker Hotel, and I am joined by Dr. Jeff Smith, the Chief Research Officer at Precision BioSciences. Jeff, it's good to have you here.
Jeff Smith
Thank you. I’m excited to walk through the exciting story that is Precision BioSciences.
So taking you all the way back, my background has always been in gene editing. As a graduate student, I helped develop some of the first gene editors, customizable gene editors called Zinc Finger Nucleases. And I helped to develop, actually, in collaboration, some of the first examples of in vivo gene editing.
And then I went from Johns Hopkins to Duke University, where I did my post doctoral fellowship. There, I joined up with a good friend of mine from Johns Hopkins that also worked on the gene editors, and we still had a passion for figuring it out, and we knew the limitations.
We wanted to come up with a better tool, and so we set to it. After about two years at Duke, we actually invented ARCUS, which is the foundational technology for Precision BioSciences. The impetus was coming up with a gene editing technology that we knew overcame some limitations we knew currently in the field.
From there, we rolled out of Duke University. It is sort of funny, but we largely started at a 2 year old’s birthday party. The other co-founder sat down at a table and met somebody who was an MBA looking for an idea, and we were looking for somebody to help roll out the company. And that's how we ended up generating the first team that rolled out the technology.
A precise platform for clinical gene editing
Precision BioSciences is transforming drug development for new, potentially first- and best-in-class cell and gene therapies with its proprietary ARCUS gene-editing platform, which is derived from a small, naturally occurring DNA-editing nuclease.
A little-known gene editor, tested with help from a disgraced gene therapist seeking redemption, may have cured a 1-year-old boy of a deadly metabolic disorder. Announced last week by a company developing the therapy, the result could be the first success at stitching a curative gene into a “safe harbor,” a specific chromosomal location where its integration is unlikely to disrupt existing DNA in a way that triggers cancer or other problems. Because the gene should now be integrated in the baby’s genome, in this case within cells of the boy’s liver, it should persist as the organ—and person—grows.
The gene editor, dubbed ARCUS, is a DNA-cutting enzyme known as a nuclease. It is in some ways simpler and potentially better than the more famous CRISPR plat- form and could also help treat other genetic metabolic disorders. The company, iECURE, will not present data for the treated infant until March. But the apparent success of the safe harbor approach with the editor in the very first patient who received it is especially significant for iECURE co-founder James Wilson, who helped develop the new treatment.
Summary
Earlier this year iECURE reported that an infant with the rare and deadly liver disease OTC Deficiency had a complete response to its experimental gene editing therapy. It is believed to be the first time that an infant was treated with an in vivo, liver directed gene editor. The treatment restored ammonia levels in the child’s blood to normal, and the child is off of ammonia scavenger medicines and is eating a normal diet. We spoke to Joe Truitt, CEO of iECURE, about the company’s experimental therapy for OTC Deficiency, how it works, and its approach to in vivo editors.
Daniel Levine
I'm Daniel Levine, and this is Rarecast. Earlier this year, iECURE reported that an infant with the rare and deadly liver disease OTC Deficiency, had a complete response to its experimental gene editing therapy. It's believed to be the first time that an infant was treated with an in vivo liver directed gene editor. The treatment restored ammonia levels in the child's blood to normal, and the child is off of ammonia scavenger medicines and is eating a normal diet. We spoke to Joe Truitt, CEO of iECURE, about the company's experimental therapy for OTC, how it works, and its approach to in vivo gene editors. Joe, thanks for joining us.
Joe Truitt, CEO of iECURE
Well, thank you for having me.
Daniel Levine
We're going to talk about gene editing, iECURE, and its efforts to advance in vivo gene editing therapies for people with rare liver diseases. A lot has happened since we first featured the company in 2021 shortly after it was unveiled. The company grew out of a collaboration with Jim Wilson at the University of Pennsylvania's Gene Therapy Program. Can you remind listeners about the origins of iECURE and its relationship with the Gene Therapy Program?
Joe Truitt, CEO of iECURE
Absolutely. Jim Wilson had been working on how to address these errors of inborn metabolism for years at the University of Pennsylvania, and he finally landed on a construct that was working in the animal models. He completed his mouse work, and then they moved on into the non-human primates. And after the non-human primate data came back with very promising results, Jim took that out to investors, to venture capital investors, and then they got excited about the data and decided to form a company. And that's when, when I joined the group as part of the original management team to take Jim's science and move it into the clinic.
Maury Raycroft
My name is Maury Raycroft and one of the biotechnology analysts at Jefferies. With great pleasure, and I would like to welcome Michael Amoroso and Dr. Cassie Gorsuch from Precision BioSciences who are joining us today for a fireside chat. Thanks so much for being here. Michael, 2025 is turning out to be a big year for Precision BioSciences on multiple fronts. Please provide an introduction to Precision’s refocus on the in vivo gene editing platform, and then we'll dig into some of the details.
Michael Amoroso, Precision BioSciences, CEO
Welcome to the audience. Maury, thank you for having us. Precision Biosciences is out of Durham, North Carolina. ARCUS is our proprietary gene editing platform. Precision BioSciences is an in vivo gene editing company, and it is very exciting right now as we are in the clinic. In 2025 into 2026, we have three clinical milestones that will be very important.
With ARCUS, which is our in vivo gene editing technology, as we speak, we are in the clinic, and we showed some data in March 2025 from our lead trial ELIMINATE–B for chronic hepatitis B. We are in Phase 1 in the safety and efficacy and dose-establishing part of the schedule. This is our first trial, and it is wholly owned.
Second, at the end of the year, we will file an IND and/or CTA for Duchenne Muscular Dystrophy. We just announced that program, and we are going to be going into the clinic in early 2026. And we also have a partner program, the DMD programs, also wholly owned. We also have a partner program for OTC Deficiency, a rare disease, a deadly disease, unfortunately, in infants. Our partner out of University of Pennsylvania, iECURE is in the clinic right now in the OTC–HOPE trial for OTC deficiency.1
Patrick Trucchio
Welcome to the 1st Annual H.C. Wainwright Virtual Genetic Medicines Conference. My name is Patrick Trucchio. I am a Senior Health Care analyst at HCW. Together with my colleagues, we are pleased to be hosting some of the most innovative biotechnology companies in the world alongside leading key opinion leaders in areas of gene editing, RNA medicines and gene therapy. We are excited to feature a broad mix of established and emerging players to highlight the next generation approaches advancing genetic medicine. With that, it is my pleasure to introduce our next speakers, Precision BioSciences Co–Founder and Chief Research Officer Jeff Smith and Chief Science Officer, Cassie Gorsuch.
Precision BioSciences is a clinical stage biotechnology company using its proprietary ARCUS gene editing platform to pioneer development of precision medicines. While Precision BioSciences’ lead program PBGENE–HBV is advancing in a Phase One trial for treatment of chronic HBV infection, today's discussion will focus on PBGENE–DMD, a differentiated program designed to restore near muscular function for Duchenne Muscular Dystrophy.
To start, let's begin with Precision BioSciences’ ARCUS gene editing platform. This is a unique platform that you are using to develop in vivo gene editing therapies targeting the root cause of rare genetic disease as well, as well as propelling infectious disease. What makes it distinct from other gene editing approaches?
Dr. Jeff Smith
So really, it boils down to the difference in origin. My entire career, nearly 30 years, has been in the field of gene editing and developing gene editing tools. I was fortunate enough to be involved in some of the first gene editing tool development, and I learned from that experience, basically some characteristics that would be involved in an ideal gene editor.
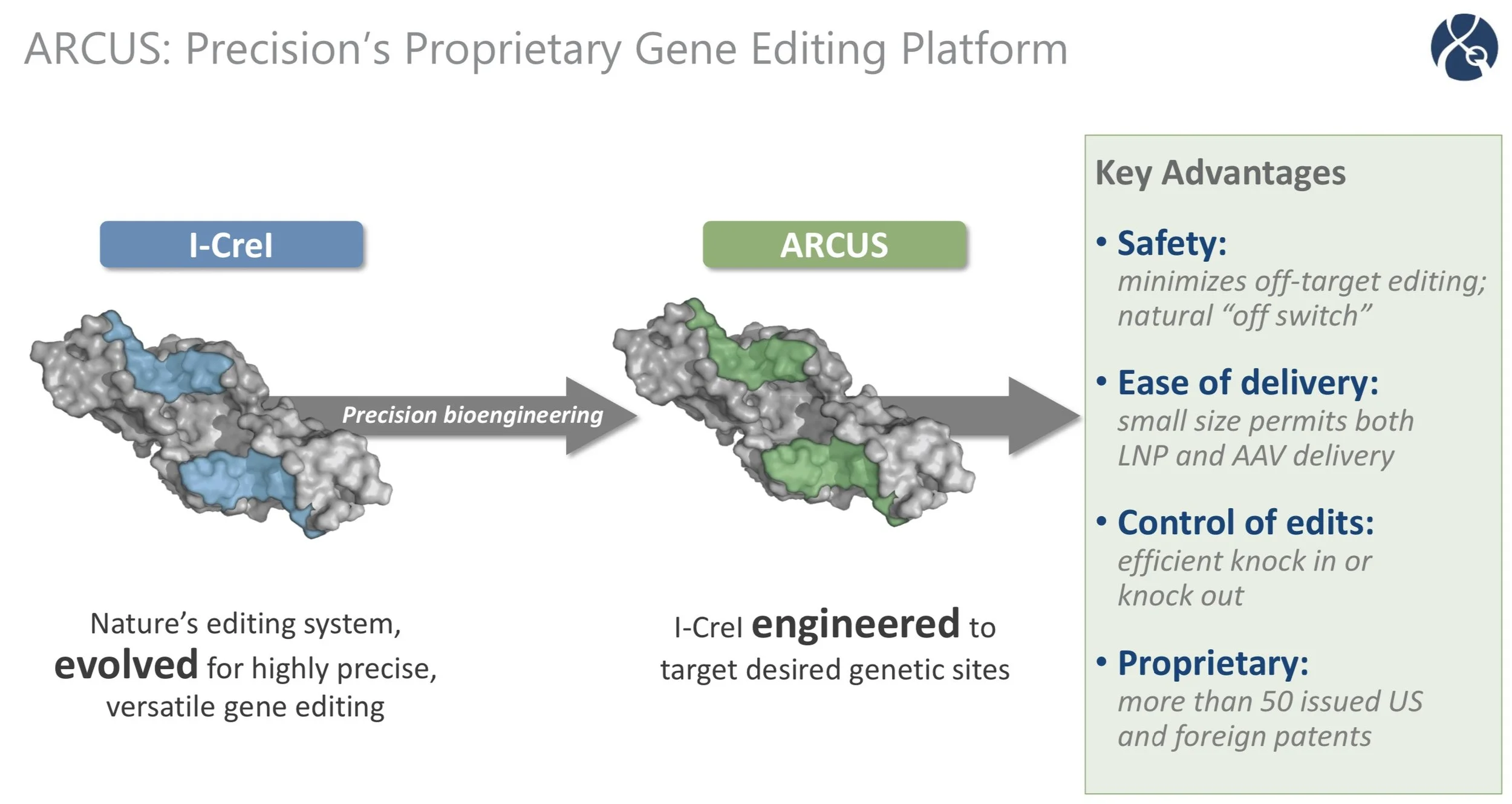
After pursuing trying to build our own gene editing platform, we ended up with ARCUS. ARCUS is unique from all the other gene editors, in that it is derived from a class of enzymes called homing endonucleases, and not based off of CRISPR, which is sort of the basis for many of the other gene editors in the field.
This difference in origin helps bring some key differentiating advantages. The first being that ARCUS generates this staggered cut. Actually, we just published a paper in Nucleic Acids Research that highlights the importance of that cut in being able to drive efficient cutting and as well as certain types of repair (homology directed repair).
And then a second advantage has been size. ARCUS is by far the smallest gene editor, and that helps, in particular, with delivery.
And then the third is what we call simplicity. ARCUS is a single component gene editor. You do not have to deliver multiple components, and this really helps with both efficacy as well as delivery.
Moderator: Geulah Livshits, Ph.D. Chardan Senior Biotechnology Research Analyst
Erik Aznauryan, Ph.D. HarborSite, Co-Founder and CEO
Albert Seymour, Ph.D. Seamless Therapeutics, Chief Executive Officer
Dr. Gabriel Cohn, iECURE, Chief Medical Officer
Dr. Cassie Gorsuch, Precision BioSciences, Chief Scientific Officer
Welcome to this session focusing on new applications of genome editing technologies.
I am Geulah Livshits, a biotechnology analyst at Chardon. Over the past 10 years, gene editing technologies, and most notably CRISPR-Cas9 nucleases, drew a lot of attention for their ability to be readily programmed to cut specific stretches of DNA, to disrupt genes or regulatory regions in the genome as a way to durably silence or upregulate a gene as we have seen for Casgevy. And this was accelerated by the pretty straightforward programmability of the guide RNA component.
But in the last several years, we have also seen major leaps in protein engineering andysis, and those have enabled scientists to advance other non CRISPR nucleases and optimize other DNA modifying enzymes to expand the applications of editing technologies. So in this session, we will discuss how applications of some of these technologies for gene insertion or making larger changes in the genome and for targeting viral genomes can be deployed.
I am joined by our participants: Erik Aznauryan, Co-Founder and CEO of HarborSite, Dr. Gabriel Cohn, Chief Medical Officer of iECURE, and Dr. Cassie Gorsuch, Chief Science Officer of Precision BioSciences, and Albert Seymore, CEO of Seamless Therapeutics.
Thank you all for joining us today, and let's start the session by having each of our participants briefly introduce themselves and their company's technology and programs. And let's start here on this end.
Dr. Luis Santos
Good morning. And Welcome to the 1st Annual H.C. Wainwright Virtual Liver Disease Conference. I am Luis Santos, a healthcare associate at H.C. Wainwright. This year, for the first time, we are combining our Viral Hepatitis and M.A.S.H. Conferences. This way, we expanded the scope to also include liver cancer. And we are very pleased to be hosting some of the most innovative biotechnology companies in the world alongside key European leaders in the areas of Hepatitis B virus, Hepatitis Delta virus, and M.A.S.H. and other treatments. And with that, I want to introduce our next speaker, Dr. Cassie Gorsuch, the Chief Scientific Officer of Precision BioSciences, a clinical stage biotechnology company pioneering the ARCUS gene editing platform to develop in vivo gene editing therapies that target the root cause of genetic and infectious diseases. Cassie, welcome. It is a pleasure to have you.
Dr. Cassie Gorsuch
Thank you so much for having us. I am happy to be here.
Dr. Luis Santos
For this fireside chat, we are going over the usual suspects: We are going to start with an introduction to your platform, ARCUS; going through the mechanism of action in your lead program in Hepatitis B Virus; the scientific rationale for that mechanism; the data generated; and how do you see this going forward? Can you give us a brief introduction on your ARCUS platform, and explain how this is different from other gene editing technologies?
Dr. Cassie Gorsuch
Yes, I would be happy to. At Precision BioSciences, we have a proprietary gene editing platform that we call ARCUS. We developed this gene editing platform. It has some pretty distinct features that we think make it a really great basis for therapeutic gene editing.
It is quite different from the other gene editing technologies that others may be familiar with, like CRISPR–Cas9 technology. So our ARCUS platform, the nuclease is actually derived from a protein that occurs in nature in a green algae. Our founders spent the better part of 10 years determining how to take that protein that occurs in nature and adapt it and engineer it to be able to recognize a target sequence of our choice and really hone the specificity through protein engineering.
As I mentioned, it has some unique features that I am sure we will get into here in a minute. We think this technology has the potential to be leveraged in a number of different therapeutic applications to provide differentiated types of editing outcomes that really matter when you use gene editing therapeutically.
That is our platform. We own the ARCUS technology. We develop those nucleases, and that is a consistent thread across all of our portfolio is the use of an ARCUS nuclease in in vivo gene editing.
Moderator: Cassie Gorsuch, Ph.D. Chief Scientific Officer, Precision BioSciences
Featuring: Pat Furlong, President, Parent Project Muscular Dystrophy
Aravindhan Veerapandiyan, M.D. (Dr. Panda)
Associate Professor of Pediatrics in the Division of Pediatric Neurology
DMD Clinical Investigator at the University of Arkansas for Medical Sciences at Arkansas Children’s Hospital
Discussion Topics
● The unmet medical need in Duchenne Muscular Dystrophy
● The science behind ARCUS gene editing technology
● Precision BioSciences’ roadmap toward IND and CTA filings in 2025
● The path to anticipated clinical data in 2026
This event will offer a detailed look at the science, mission, and future vision driving PBGENE–DMD and how Precision BioSciences is advancing gene editing to deliver meaningful, long-term impact for patients and families affected by Duchenne Muscular Dystrophy.
Cassie Gorsuch, Ph.D. — Chief Scientific Officer
I am joined today by two well known folks in the field of muscular dystrophy, Pat Furlong, the President at Parent Project Muscular Dystrophy (PPMD), and Dr. Veerapandiyan, a pediatric neurologist at the Arkansas Children’s Hospital. I am going to give each of them an opportunity to introduce themselves and tell you a little bit more about who they are.
Michael Amoroso — President & Chief Executive Officer
Welcome to our investment community today. It is wonderful to be with you on the back of AASLD, where we showed our late breaking data last evening. My name is Michael Amoroso, and I am the Chief Executive Officer, with the privilege to lead this team.
ARCUS is no longer just a vision or a preclinical platform. The ARCUS platform is the underlying backbone of Precision BioSciences. ARCUS is now delivering in the clinic.
First, you will see our partnered programs. ECUR–506 in the dire disease of OTC Deficiency. I will remind the investment community, it is the first patient treated this year on a gene insertion program. Proof of gene insertion of ARCUS through our partner at iECURE — Joe Truitt and team. The first patient is now over a year and a half old in a complete response.
10% OF ALL BLUE ROOM REVENUES GO DIRECTLY TO FUND OUR NON PROFIT TOGETHERISM.
WE CAN ACCOMPLISH ANYTHING TOGETHER.
These materials do not purport to be all-inclusive or to contain all the information that a prospective investor may desire in considering an investment. These materials are intended merely for preliminary discussion only and may not be relied upon for making any investment decision. Any discussion or information contained in this presentation does not serve as a receipt of, or as a substitute for, personalized investment advice from Blueroom or your advisor.
This publication does not constitute an offer to sell or a solicitation to buy any securities in any fund, market sector, strategy or any other product. Investing is speculative and involves substantial risks (including, the risk of loss of the investor’s entire investment). Past performance is not indicative of future results, and there can be no assurance that the future performance of any specific investment, investment strategy, or product will be profitable.
For more information about us and our general disclosures contact us directly.